USE/CARE OF HANDTOOLS & MEASURING TOOLS - OD1621 - LESSON 2/TASK 1
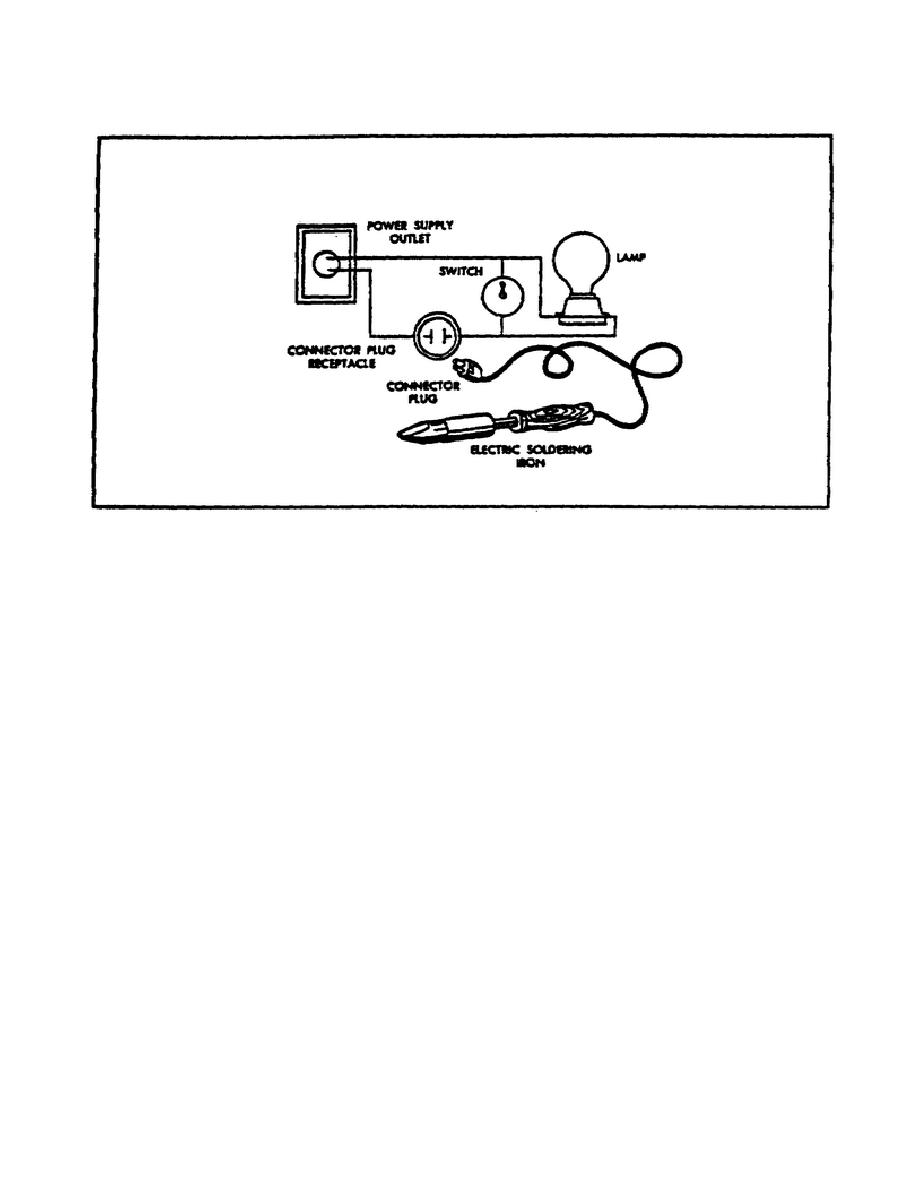
FIGURE 59.
ELECTRICAL CIRCUIT TO PREVENT OVERHEATING IRON.
(3) Using Fluxes. It is almost impossible to solder without a flux.
Metal surfaces, at the point where they are to be joined together, must be
absolutely clean and bright so that the solder will adhere to them. A thin
layer of oxide forms on bright surfaces in a few seconds, as soon as they
are heated by the iron. To remove the oxide coating, apply flux. Fluxes
are chemicals; the most common in use for soldering are rosin and zinc
chloride. Zinc chloride is an acid flux. Rosin fluxes are noncorrosive and
nonconductive and are the best fluxes to use when soldering metals, copper,
old tinware, electrical connections, radios, telephones, fine instruments,
and small parts. Zinc chloride or paste solder is corrosive and is commonly
used in heavier work on untinned copper, brass, bronze, monel metal, nickel-
plated parts, galvanized iron, zinc, steel, and German silver. Since it is
corrosive, all traces of it should be washed off the work after soldering.
The only disadvantage of using rosin flux is that rosin is adhesive.
Consequently, a joint which may appear to be well soldered may actually be
held together by the adhesive properties of the rosin and not by the solder.
To prevent this, the soldering iron must be hot enough to cause the rosin to
burn and must be held against the joint until it smokes. Be constantly on
the alert to avoid "rosin joints" when making electrical connections.
78




 Previous Page
Previous Page
