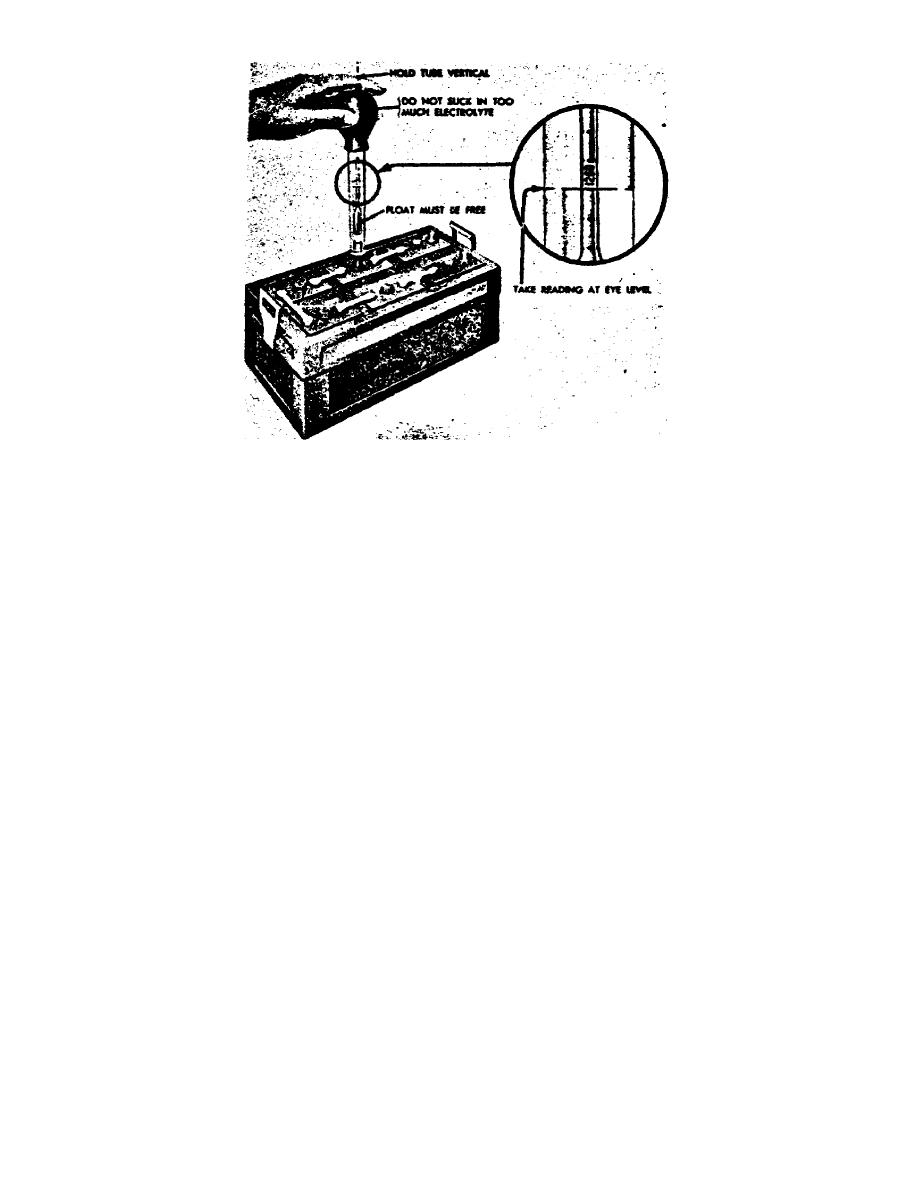
Figure 21.
Testing the specific gravity of a battery.
e. The gravity of the battery is affected by temperature. When heated, the
electrolyte expands so it occupies more space. When the temperature drops, the
electrolyte contracts and takes up less space. In view of this, warm electrolyte
will weigh less than the same volume of cool electrolyte, so warm electrolyte has a
lower specific gravity than cold electrolyte. Electrolyte that has been mixed for
normal use will test 1.280 at 80 F. This is the temperature of the electrolyte
and not the surrounding air. At ordinary temperatures it is not necessary to
consider any variations when testing the gravity of a battery. However, any large
variation above or below 80 is very important when deciding the true state of
battery charge.
f. In order to correct for temperature changes, test both the gravity and
temperature of the electrolyte. Some hydrometers have a built-in thermometer so
you can do both at the same time. For each 10 of temperature variation from 80,
change the gravity reading 4 points (fig 22). Add points to gravity readings when
the electrolyte is above 80; take points away when the temperature is below 80.
g. The electrolyte should be at the correct level in the cell when it is
tested. Water in the electrolyte evaporates, the acid does not. If the water has
evaporated enough so the electrolyte level is low, the mixture will be strong and
the gravity reading will be high. On the other hand, if the battery has been
overfilled with water the electrolyte will be weakened and the reading will be low.
When water is added, it will tend to remain at the top of the cell so a hydrometer
reading taken immediately after adding water would not be right. If water has to
be added before taking a reading, the battery should be charged for 1 to 2 hours to
mix the electrolyte before the hydrometer is used. This may be done by connecting
the battery to a battery charger. Gravity readings will not tell the true state of
the electrolyte if taken Just after a battery has been discharged at a high rate.
The acid has been used up next to the plates but the electrolyte near the top of
the plates is still strong. The hydrometer will read a higher state of charge than
that which actually exists. The electrolyte will mix so a true reading can be
obtained if the battery is allowed to stand unused for several hours or if it is
charged for 1 to 2 hours.
OS 010, 3-P26




 Previous Page
Previous Page
