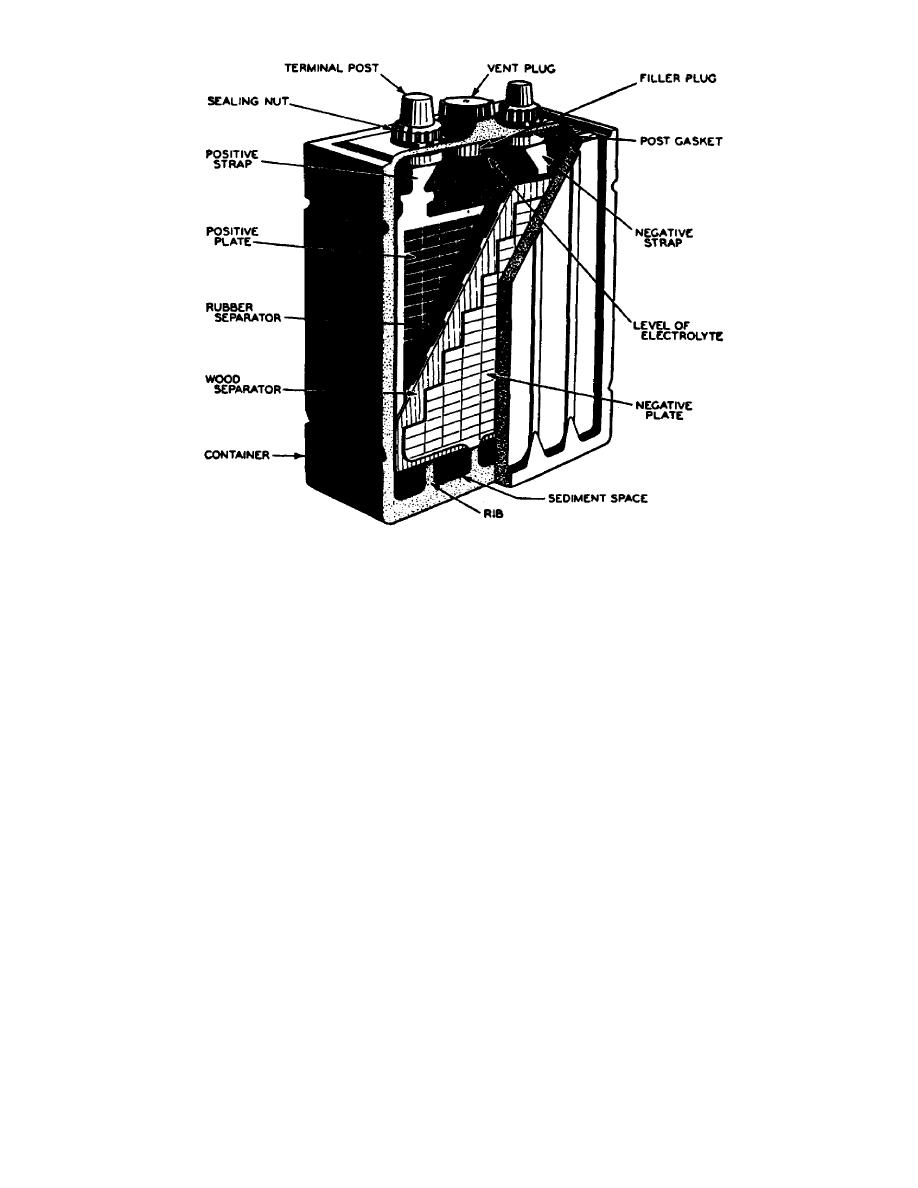
Figure 16.
Cutaway view of single cell.
e. The top of each cell is fitted with a cell cover made from the same
material as the container. Some batteries have cell covers made so that straps
used to connect the cell terminals are exposed. On others, the connecting straps
are covered and only two terminal posts are exposed. Regardless of the type of
cover used, each cell cover is fitted with a vent plug which may be removed. to
inspect the cell or to add water. To seal the battery after the cell cover is
installed, the space between the edges of the cell covers and the container is
filled with an acid-resistant battery sealing compound. Some form of seal is also
used where the terminal post extends through the cover. The terminal posts are the
output connections of the battery. The terminals are tapered and the positive
terminal is slightly larger than the negative terminal (fig 17).
12. LEAD-ACID BATTERIES (CHEMICAL ACTION).
a. When a load such as a lamp is connected to the battery, electrons flow
through the lamp from the negative plates to the positive plates. At this time,
the battery is said to be discharging. Several chemical changes take place inside
the battery during the discharge (fig 18). As the current flows, atoms of the
sulfuric acid leave the electrolyte and enter the battery plates. This decreases
the amount of acid that is in the electrolyte making the mixture weaker. As you
may recall, atoms from all elements are different and the nature of all materials
is determined by the atoms they contain. Therefore, the atoms of sulfuric acid
mixing with the battery plates will change the nature of the plates. The spongy
lead of the negative plates turns into lead sulphate; the lead peroxide of the
positive plates also turns into lead sulphate.
OS 010, 3-P22




 Previous Page
Previous Page
