BASIC ELECTRONICS - OD1633 - LESSON 1/TASK 1
FIGURE 1.
COMPOSITION OF MATTER.
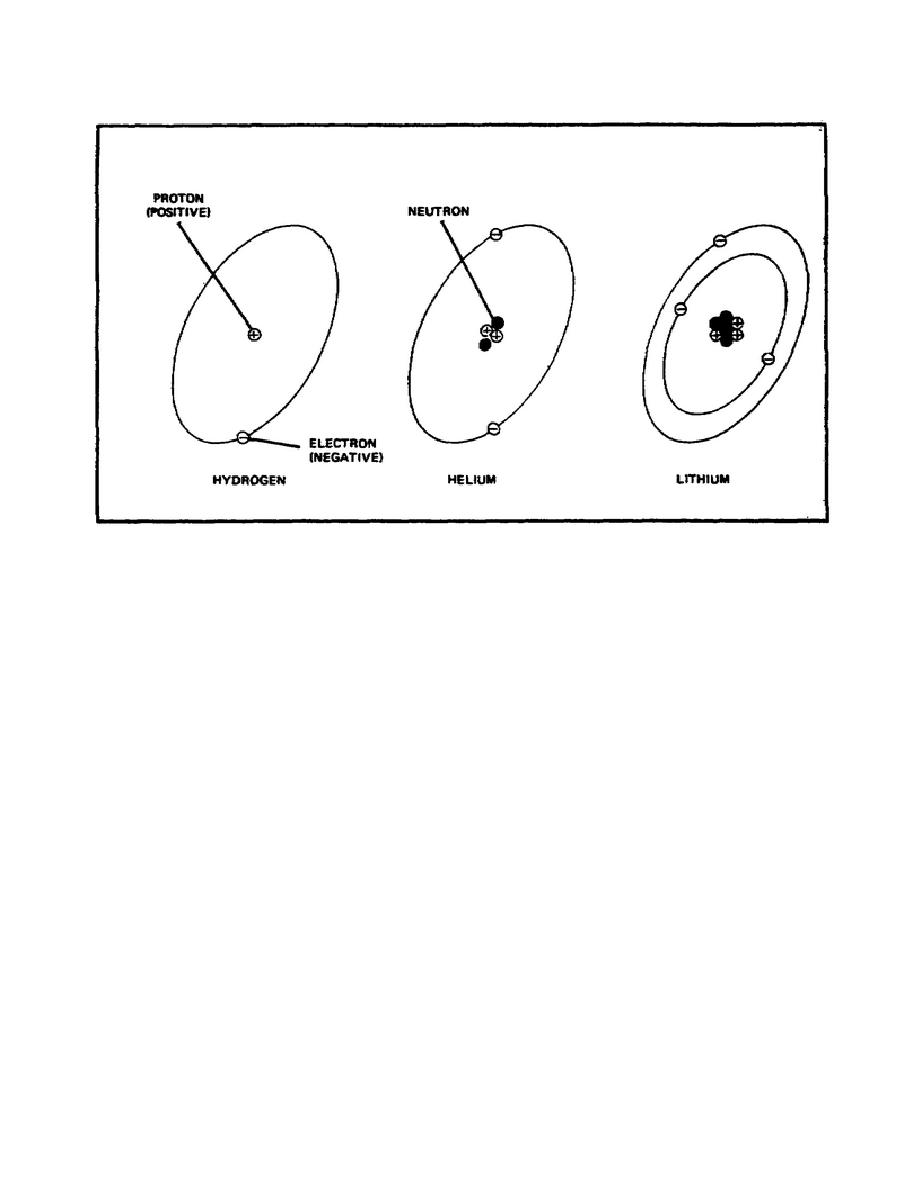
A still more complex atom, shown in figure 1, is the atom of lithium, a
light, soft metal. Note that a third proton has been added to the nucleus
and that a third electron is now circling around the nucleus.
There are
also two additional neutrons in the nucleus; these are needed to hold the
three protons together.
The atoms of other elements can be analyzed in a similar manner.
As the
atomic scale increases in complexity, protons and neutrons are added one by
one to the nucleus and electrons to the outer circles or shells, as they are
termed by scientists. After lithium comes beryllium with four protons and
five neutrons, boron with five protons and five neutrons, carbon with six
and six, nitrogen with seven and seven, oxygen with eight and eight, and so
on.
In each of these, there are normally the same number of electrons
circling the nucleus as there are protons in the nucleus.
b. Composition of Electricity. When there are more than two electrons
in an atom, they will move about the nucleus in different size shells. The
innermost shells of the atom contain electrons that are not easily freed and
are referred to as bound electrons. The outermost shell will contain what
are referred to as free electrons (see figure 2 on the following page).
These free electrons differ
4




 Previous Page
Previous Page
