ELECTRONIC PRINCIPLES - OD1647 - LESSON 1/TASK 1
electron also revolves on its axis as it orbits the nucleus of
an atom.
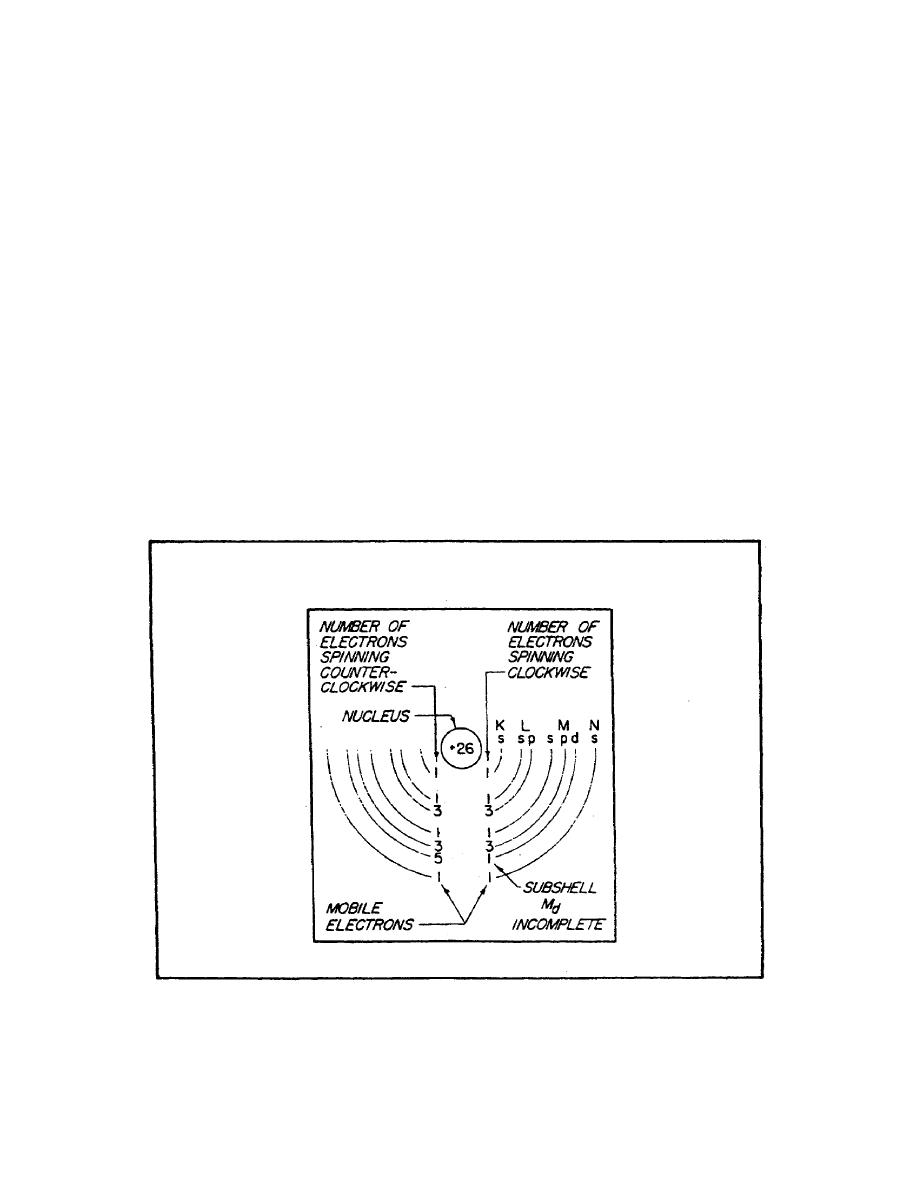
It has been experimentally proven that an electron has a
magnetic field about it, together with an electric field. The
effectiveness of the magnetic field of an atom is determined by
the number of electrons spinning in each direction.. If an atom
has equal numbers of electrons spinning in opposite directions,
the magnetic fields surrounding the electrons cancel one
another, and the atom is unmagnetized. However, if more
electrons spin in one direction than another, the atom is
magnetized. An atom with an atomic number of 26, such as iron,
has 26 protons in the nucleus and 26 revolving electrons
orbiting its nucleus. If 13 electrons are spinning in a
clockwise direction and 13 electrons are spinning in a
counterclockwise direction, the opposing magnetic fields will be
∙neutralized. When more than 13 electrons spin in either
direction, the atom is magnetized. An example of a magnetized
atom of iron is shown in figure 2.
FIGURE 2. IRON ATOM (DOMAIN THEORY).
7





 Previous Page
Previous Page
