Lesson 1/Learning Event 1
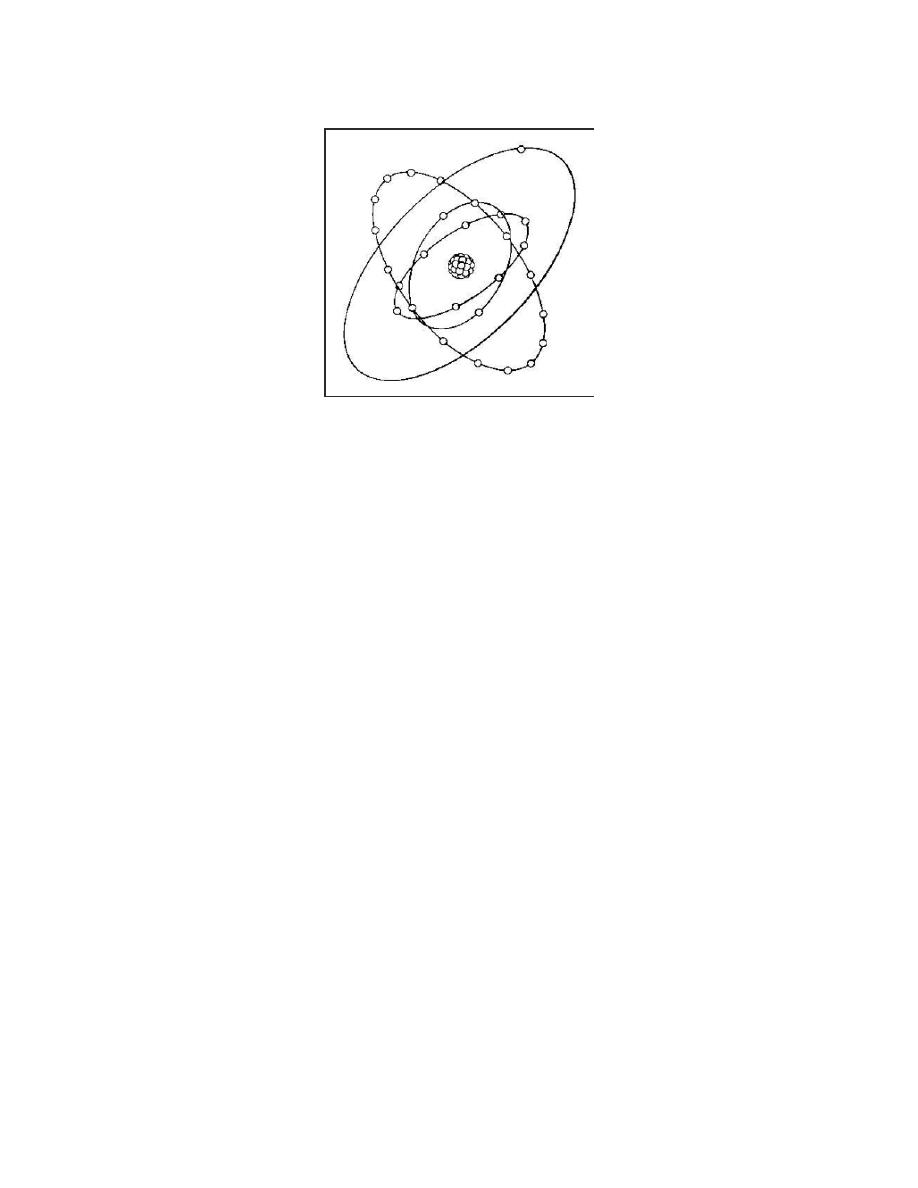
FIGURE 1. COPPER ATOM.
As an example, we will use one atom of the element "copper." Imagine that we have a magnifying
glass that is able to magnify the atom so it appears to be one hundred million times larger than its
actual size. This makes it look to be about 1 inch in diameter. We can now easily see that the
atom has a central body, the nucleus. We can also see there are 29 smaller particles--electrons--
rapidly circling around the nucleus, each one moving in a different path.
Now, imagine that we are able to magnify this copper atom even more, so that only the nucleus
fills the viewing area. The nucleus now looks like a bunch of grapes. It actually consists of 64
particles bound tightly together, 29 of which are protons and the other 35 are neutrons.
All atoms in any one element are exactly alike. They have the same number of electrons, protons,
and neutrons; they are all the same size; and all weigh the same. On the other hand, atoms from
different elements are completely different. The atoms from each element contain an equal
number of electrons and protons. Recall that our copper atom contains 29 electrons and 29
protons.
5





 Previous Page
Previous Page
